
Non-Animal & Human-Relevant Research News: November 2025
While the animal research industry continues to breed, buy, cage, torture, and kill sentient beings, progressive scientists are busy proving that human-relevant science is not only possible but, in fact, better for us all. Highlights in science from the last month are below.
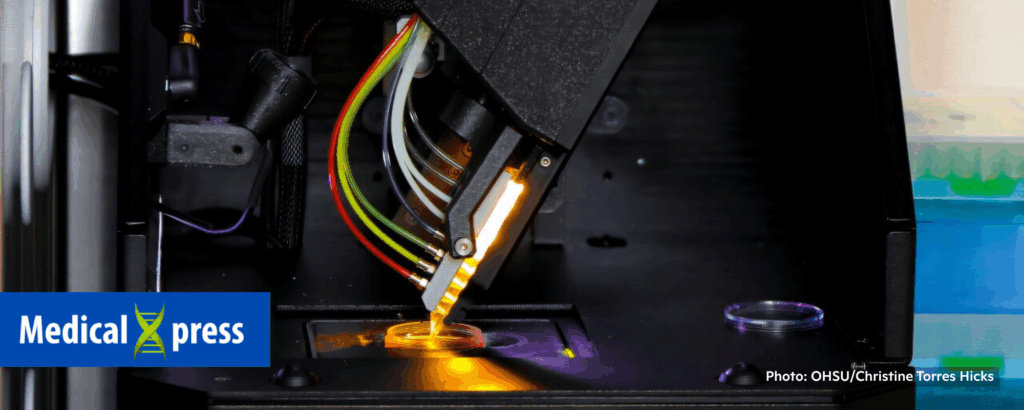 How new advances in 3D bioprinting, organoids and organs-on-a-chip aid in the fight against cancer
How new advances in 3D bioprinting, organoids and organs-on-a-chip aid in the fight against cancer
Oregon Health & Science University, Medical Xpress, 11/3/2025
“A new review by researchers at Oregon Health & Science University’s Knight Cancer Institute and other universities highlights how advances in New Approach Methodologies and tissue engineering are offering powerful new tools to study the earliest stages of cancer development.”
“New Approach Methodologies use human-relevant technologies such as in vitro tests, organoids, organs-on-a-chip and computational modeling to replace, reduce or refine animal testing. These lab-grown models replicate the environment inside the human body and could unlock clues about how cancer begins.”
“Despite years of cancer research, scientists know relatively little about what happens in the body during the early stages of cancer . . . These models, which have recently been prioritized as so-called New Approach Methodologies for medical research, let researchers precisely recreate and manipulate the early tumor environment, allowing them to test how specific cellular, genetic or environmental factors influence cancer development. This approach also supports the discovery of new biomarkers—biological red flags that could help clinicians detect cancer earlier and more accurately.” 📰 Full Story →
 Charles River to cut costs, shift direction as revenue slide continues
Charles River to cut costs, shift direction as revenue slide continues
Darren Incorvaia, Fierce Biotech, 11/5/2025
“The board of Charles River Laboratories has completed a strategic review of the company, deciding to cut costs and shift investments into animal testing alternatives and other potential areas of growth . . . Charles River will invest in growth areas such as bioanalysis and new approach methodologies (NAMs), which are alternatives to animal testing” and “include tools like organoids, organs-on-chips and computer models that simulate biology.”
“The research outfit’s investment in NAMs comes as no surprise; Charles River’s interest in animal testing alternatives predates the second Trump administration, but the vocal support for NAMs from the federal government this year has reinvigorated the CRO’s efforts. Charles River tapped Namandjé Bumpus, Ph.D., former principal deputy commissioner of the FDA, to lead a scientific advisory board guiding NAM strategy in October. The company is also an active participant in the Validation and Qualification Network, an initiative of the Foundation for the National Institutes of Health that brings together industry and regulators to help get the most promising NAM technology across the regulatory finish line.” 📰 Full Story →
 How Certara turned the FDA’s surprising decision on animal testing into a launchpad for innovation
How Certara turned the FDA’s surprising decision on animal testing into a launchpad for innovation
Karuga Koinange, Technical.ly, 11/6/2025
“Within days of the FDA’s announcement, Certara launched the Non-Animal Navigator (NAM) — an artificial intelligence platform designed to help pharmaceutical companies replace or reduce animal testing. Certara also created a dedicated website for this service, positioning itself as the first major company offering a comprehensive platform to guide pharmaceutical developers through the post-animal testing era. And investors took notice: Certara’s stock jumped nearly 30% that week.”
“Certara’s Non-Animal Navigator combines in vitro methods, organ-on-a-chip assays, biosimulation and regulatory strategy into one streamlined solution. It essentially provides a step-by-step framework for identifying when and how non-animal methods can replace animal studies while ensuring alignment with FDA expectations.” 📰 Full Story →
 3D Bioprinted Melanoma Models Revolutionize Cancer Therapy
3D Bioprinted Melanoma Models Revolutionize Cancer Therapy
Scienmag, 11/6/2025
“In recent years, malignant melanoma has persisted as one of the deadliest forms of skin cancer, continuously challenging researchers and clinicians alike due to its aggressive progression and frequent resistance to conventional therapies. The complexity of melanoma, especially its interaction within the tumor microenvironment, calls for sophisticated and reliable models that can accurately replicate human skin and tumor biology. Traditional two-dimensional (2D) cell cultures and even standard three-dimensional (3D) systems such as spheroids and organoids, though useful, fail to comprehensively simulate the multi-layered, vascularized, and immunologically active environment of native skin. This gap has driven the development of advanced platforms, among which 3D bioprinting emerges as a revolutionary technology enabling the precise construction of melanoma models that hold promise for both understanding tumor dynamics and screening innovative therapies.”
“One of the most compelling applications of these 3D bioprinted melanoma models lies in their utility for assessing anticancer strategies….”
“In summary, the integration of bioprinting technology with melanoma research marks a formidable advance, offering robust platforms that recapitulate native skin conditions and tumor microenvironments with unprecedented precision . . . As these technologies mature, they have the potential to transform both experimental oncology and personalized medicine, providing new hope against one of the most lethal forms of skin cancer.” 📰 Full Story →
 Glasgow scientists create first lab-grown bone marrow to study cancer
Glasgow scientists create first lab-grown bone marrow to study cancer
Christina O’Neill, stv News, 11/6/2025
“Scientists at the University of Glasgow have developed the first bioengineered bone marrow model and used it to study cancer. The breakthrough could transform how new blood cancer treatments are tested and reduce the need for animal research.”
“Traditionally, testing new [“acute myeloid leukaemia (AML), the most common leukaemia in adults”] therapies has relied heavily on animals, because once taken out of the body, bone-marrow stem cells quickly die or behave differently, making lab experiments unreliable. To overcome this, the Glasgow team used human cells combined with hydrogels – jelly-like materials – to recreate the complex structure of bone marrow in the lab. They then introduced leukaemic stem cells into the model and tested CAR T-cell therapy against them.”
“‘Our results highlight the potential of non-animal technologies for studying and developing new leukaemia therapies. This approach could reduce reliance on animal models in drug testing over time, ultimately paving the way for more efficient and effective development of therapies for patients.’” 📰 Full Story →
 The Next Superintelligence Will Not Just Think. It Will Bleed.
The Next Superintelligence Will Not Just Think. It Will Bleed.
Julie O’Shaughnessy, Genetic Engineering & Biotechnology News, 11/13/2025
“Biological data centers of living human tissue give AI the feedback animal models never could.”
“Biology needs an environment that gives intelligence the same systematic feedback that data centers gave to computation. That is what biological data centers provide. Robotic systems that sustain tens of thousands of standardized human tissues at once. Tissues that are vascularized and immune competent, clinically indistinguishable from patient biopsies under blinded review. Tissues that can be dosed, that bleed, that heal. With that substrate, intelligence can move beyond proxies. Molecules generated upstream can be stress tested downstream in human systems. Doses can be chosen based on human-relevant endpoints. Toxicities can be caught before an investigational new drug (IND) application is filed. Diversity is built in, with donor-derived tissues reflecting women of childbearing age, pediatric populations, and rare subtypes. Clinical trials remain. But they become confirmatory rather than exploratory.”
“Ethics and economics are converging. Patients are less willing to accept a system where animal success is treated as predictive. Investors are less willing to fund late-stage attrition. Better evidence earlier is both humane and efficient.” 📰 Full Story →
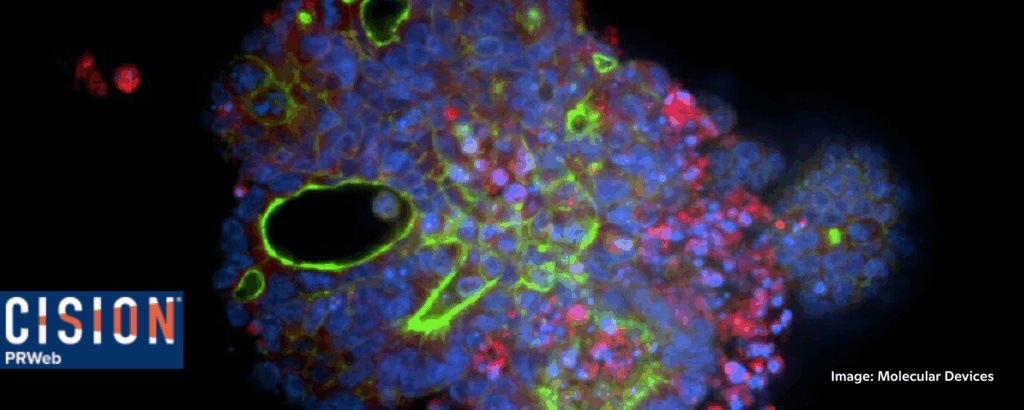 Molecular Devices Secures U.S. Patent for Scalable, Standardized Organoid Culture Method
Molecular Devices Secures U.S. Patent for Scalable, Standardized Organoid Culture Method
Molecular Devices, CISOIN PRWeb, 11/13/2025
“Molecular Devices, LLC., a leading provider of high-performance life science solutions, today announced the issuance of U.S. Patent No. 12,351,820 B2, titled ‘Methods for Culturing Organoids,’ which ‘marks an important step toward industrializing organoid research,’ said Mary Duseau, President of Molecular Devices. ‘By transforming what was once an academic process into a scalable, quality-controlled workflow, we’re helping biopharma organizations accelerate discovery while aligning with the FDA’s guidance to adopt more human-relevant, new approach methodologies (NAMs). Our goal is to give scientists confidence in consistent, assay-ready models that truly reflect patient biology.’” 📰 Full Story →
 Scientists engineer first fully synthetic brain tissue model
Scientists engineer first fully synthetic brain tissue model
Jules Bernstein, UC Riverside, 11/17/2025
“For the first time, scientists have grown functional, brain-like tissue without using any animal-derived materials or added biological coatings. The development opens the door to more controlled and humane neurological drug testing.”
“‘One of the drawbacks of most brain tissue platforms is that they utilize biological coatings to help living cells thrive. These animal-derived coatings are poorly defined, which makes it difficult to recreate their exact composition for reliable testing,’ said Iman Noshadi, a UCR associate professor of bioengineering who led the team. In addition, using animal brains to conduct research relevant to human conditions — as is currently the norm — is not ideal. There are significant genetic and physiological differences between rodent and human brains. This platform could reduce, and in some cases eliminate, the need to use animal brains for this purpose….” 📰 Full Story →
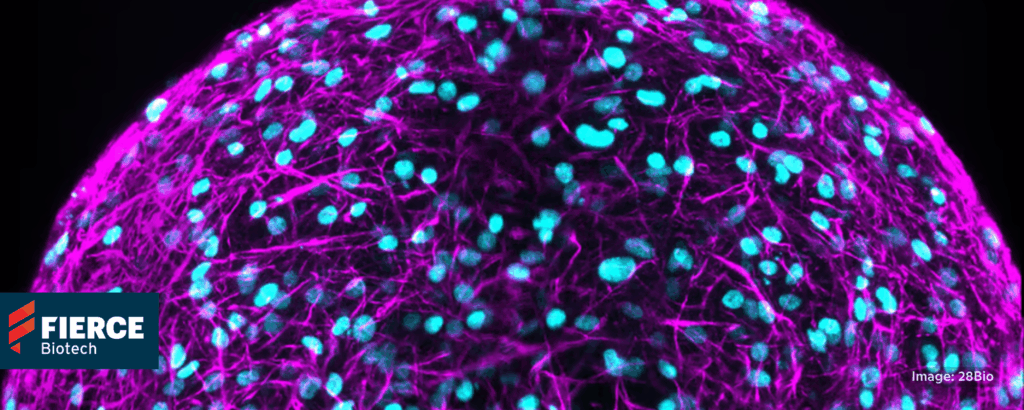 Regulatory momentum builds for organoid neurotoxicity testing
Regulatory momentum builds for organoid neurotoxicity testing
28bio, Fierce Biotech, 11/17/2025
“One in four safety-related failures are due to central nervous system (CNS) toxicity . . . Most CNS safety testing still relies on animal behavioral studies or 2D cell culture cytotoxicity assays, endpoints that poorly correlate to the functional network disruptions clinicians see in patients . . . [The “shift” in U.S. policy] reflects growing recognition that human biology cannot be faithfully modeled in animals, particularly for the CNS.”
“Human-derived brain organoids offer a more clinically predictive alternative” that “now enable clinically relevant and scalable neurotoxicity testing with mechanistic insight unattainable in animal models. Organizations that adopt these models now will identify liabilities earlier, prevent costly late-stage failures, and redirect R&D spend on candidates with the highest chance of clinical success.” 📰 Full Story →
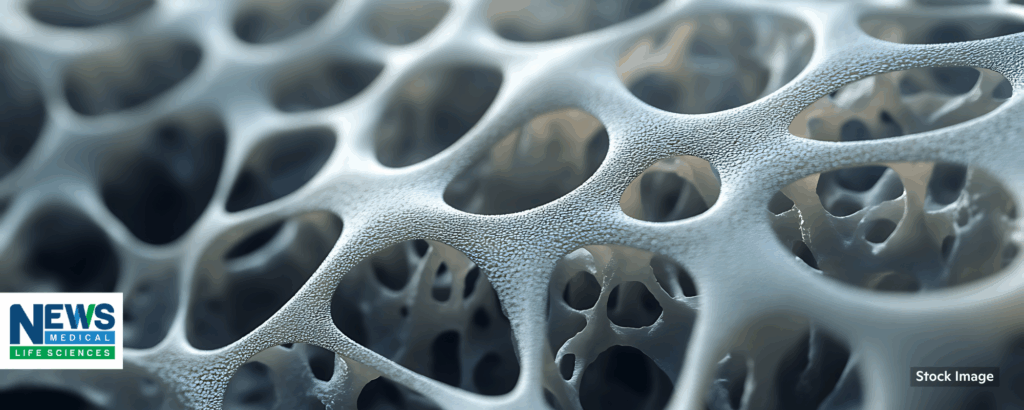 New human-cell bone marrow model offers alternative to animal experiments
New human-cell bone marrow model offers alternative to animal experiments
University of Basel, 11/18/2025
“Our body’s ‘blood factory’ consists of specialized tissue made up of bone cells, blood vessels, nerves and other cell types. Now, researchers have succeeded for the first time in recreating this cellular complexity in the laboratory using only human cells. The novel system could reduce the need for animal experiments for many applications.”
“Typically, bone marrow research relies heavily on animal models and oversimplified cell cultures in the laboratory. Now, researchers from the Department of Biomedicine at the University of Basel and University Hospital Basel have developed a realistic model of the bone marrow engineered entirely from human cells. This model may become a valuable tool not only for blood cancer research, but also for drug testing and potentially for personalized therapies….” 📰 Full Story →
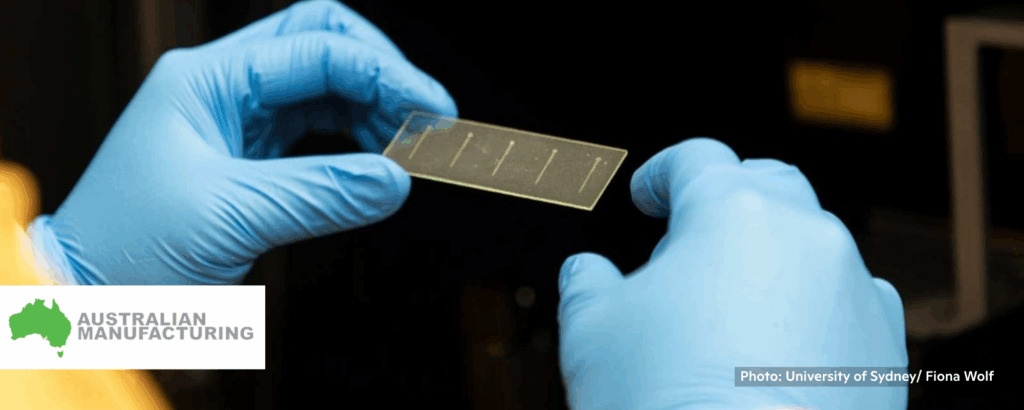 University of Sydney develops 3D printed blood vessels to aid stroke research
University of Sydney develops 3D printed blood vessels to aid stroke research
Kate B., Australian Manufacturing, 11/19/2025
“Researchers at the University of Sydney have developed a new 3D printing technique to manufacture anatomically accurate replicas of human blood vessels in just two hours, a development the university says could advance the study of strokes and the testing of new treatments.”
“‘[The “artery on a chip”] is the first-of-its-kind bioengineering endeavour in Australia, and our work is aiming to solve two crucial gaps in heart disease diagnosis and prevention, without animal testing,’ said Dr Zihao Wang, postdoctoral chief engineer of the university’s Mechanobiology and Biomechanics Laboratory (MBL).” 📰 Full Story →
 Beyond animal testing: How human data trials are reshaping drug development
Beyond animal testing: How human data trials are reshaping drug development
pharmaphorum, 11/18/2025
“More than 90% of drugs that appear safe and effective in animal studies ultimately fail once they reach human trials, and the consequences are staggering: billions of dollars wasted, delays in new therapies, and patients left without meaningful options. The pharmaceutical industry has long accepted this inefficiency as part of the process, but momentum is now building to rethink how drugs are developed.”
“One of the most promising alternatives [to animal testing] is the use of donated human organs that cannot be transplanted. Advances in organ perfusion technology – machines that pump warm, oxygenated fluid through organs to keep them functioning outside the body – allow researchers to maintain organs in a living state for hours or even days. This creates a platform where drug candidates can be introduced, responses observed, and high-resolution data collected.”
“Perfused human organs offer unique advantages. The physiological responses are likely to be far closer to those seen in patients, including subtle metabolic and toxicological pathways that animal models miss. Sampling can be conducted far more frequently and invasively than in clinical trials, enabling the capture of early warning signals long before damage would typically manifest.” 📰 Full Story →
 Why a human-first approach is the future of drug discovery
Why a human-first approach is the future of drug discovery
Bree Foster, DDN, 11/18/2025
“[ParallelBio] is pioneering a human-first approach that combines organoids, artificial intelligence (AI), and robotics to model human biology more accurately and at scale. By starting with human biology rather than animal surrogates, Parallel Bio aims to improve the predictability of drug efficacy and safety, uncover new disease targets, and ultimately reshape how medicines are discovered and developed.”
“‘Animal models may have long been the standard, but simply put, they do not work. Drugs are extensively tested for safety and efficacy in mice, only to fail 95 percent of the time in subsequent clinical trials. As a result, 110 million animals are needlessly killed every year, drug programs take over a decade and waste billions of dollars, and patients are actively harmed by misses in safety or drugs failing to treat their disease. There is no clear case to use animals, and therefore, we are moving away from that approach.’”
“‘Human-first drug discovery approaches are emerging that start with human biology at the earliest stages, rather than relying on animal models. Using immune organoids in combination with AI, researchers can create models that replicate human immune responses across diverse populations. These models are designed to evaluate potential drug targets and predict outcomes more accurately than traditional animal studies. Such platforms are intended to reduce dependence on animals in preclinical testing and to inform the design of clinical trials by providing earlier insights into safety and effectiveness. As the technology scales, the data generated could help determine which therapies are most likely to succeed in people, potentially shortening development timelines and lowering costs.” 📰 Full Story →
 Brain-on-a-Chip Revolutionizes Fight Against Deadly Encephalitis Viruses
Brain-on-a-Chip Revolutionizes Fight Against Deadly Encephalitis Viruses
Lee Ferguson, The University of Tennessee Health Science Center, 11/24/2025
“To understand and combat [“rare but devastating infections that cause fatal brain inflammation, particularly in children and older adults”], scientists have traditionally relied on mouse models. But Dr. Jonsson’s lab is testing a revolutionary alternative — a miniature, three-dimensional system that replicates the function of the human brain. Known as a brain-on-a-chip, the technology represents what the National Institutes of Health (NIH) and the Food and Drug Administration (FDA) have been calling the future of non-animal research, or ‘NAMs,’ new-approach methods. ‘This is novel technology to advance biological and therapeutic discovery,’ Dr. Jonsson said. “The human brain chip allows us to do testing in humans. Whereas otherwise, we’re limited to doing our preclinical research with mice.’” 📰 Full Story →
 Are Animals Out? How NAMS Are Changing the Rules of the Game
Are Animals Out? How NAMS Are Changing the Rules of the Game
Jennifer Smith-Parker, BioSpace, 11/26/2025
“A coordinated national effort is emerging to bring alternatives to animal testing into routine preclinical use, backed by a fresh FDA roadmap and a global coalition of scientific and industry partners . . . On the federal side, about 15 agencies are participating, with the FDA and EPA being the most prominent. From the private sector, there are more than 40 partners ranging from small methods developers to companies like Certara, which help advance NAMs, as well as end users including pharma companies, chemical companies represented by the American Chemistry Council, and nonprofits invested either in NAM development or in reducing animal use. ”
“There is growing scientific recognition that animals do not provide adequate models of human health and disease, the roadmap states.”
“Europe and Australia are ahead of the U.S. regarding NAMs, with a greater openness to accepting these methodologies, and have actively pushed these initiatives forward faster than the U.S. ” 📰 Full Story →
 Could this Aussie AI start-up phase out lab testing on animals?
Could this Aussie AI start-up phase out lab testing on animals?
Robert White, news.com.au, 11/29/2025
“A Brisbane AI start-up company has partnered with tech giant Google in hopes of solving a dark secret plaguing the drug development industry. Queensland-based biotech company Gelomics is using AI to help grow human tissues that could one day replace the mice and cell cultures that currently dominate the drug development industry. It says its system more accurately predicts how real patients will respond, potentially saving lives, money and hundreds of millions of animals.”
“‘The problem is that models are relying on animal physiology, which has fundamental differences in the biology of animals compared to humans; they process drugs differently, the toxicity profiles are different, and also the efficacy testing is completely different,’ he said. ‘So the correlation between testing results you receive from animal trials and animal experiments to actual human results is very, very little.’” 📰 Full Story →
 Take Action: Protect Macaques
Take Action: Protect Macaques
Macaques continue to face “catastrophic” population declines, driven in no small part by the U.S. animal research industry. Act now to demand that long-tailed and pig-tailed macaques be added to the list of endangered species, with full protection under the Endangered Species Act (ESA). ⚠️ Take Action Now →
Share this news compilation on X or Bluesky.
Or share this link on Facebook or anywhere else:
riseforanimals.org/news/sci-news-nov-2025