
Non-Animal & Human-Relevant Research News: September 2025
While the animal research industry continues to breed, buy, cage, torture, and kill sentient beings, progressive scientists are busy proving that human-relevant science is not only possible but, in fact, better for us all. Highlights in science from the last month are below.
 AI-driven drug discovery picks up as FDA pushes to reduce animal testing
AI-driven drug discovery picks up as FDA pushes to reduce animal testing
Sneha S K & Puyaan Singh, Reuters, 9/2/2025
“Drug developers are increasing adoption of AI technologies for discovery and safety testing to get faster and cheaper results, in line with an FDA push to reduce animal testing in the near future. Within the next three to five years, using AI and cutting back on animal testing could reduce timelines and costs by at least half, according to 11 different experts from across contract research firms, biotech companies and brokerages.”
“The new approaches are expected to ultimately lead to lower drug prices as well….” 📰 Full Story →
 Investing in the Future of Toxicology: The Rise of Non-Animal Testing Models
Investing in the Future of Toxicology: The Rise of Non-Animal Testing Models
Cyrus Cole, AInvest, 9/1/2025
“The pharmaceutical and biotech industries are undergoing a seismic shift as non-animal testing models emerge as the cornerstone of modern toxicology.”
“The non-animal toxicology testing market . . . is expected to surprise USD 4.08 billion [by 2029], fueled by advancements in organ-on-chip systems, 3D tissue models, and AI-drive in silico stimulations. These technologies address the limitations of traditional animal testing, which has a dismal 5% success rate in translating preclinical results to human approvals.”
“The transition to non-animal models offers a trifecta of advantages for investors. First, it accelerates drug development timelines: organ-on-a-chip systems can replicate human physiology with 80% accuracy, compared to 30% for animal models. Second, AI and machine learning are enhancing predictive analytics . . . Third, ethical considerations are increasingly influencing capital flows, with ESG-focused investors prioritizing companies that reduce animal use . . . The non-animal toxicology market is at a strategic inflection point, where technological innovation, regulatory clarity, and ethical demand are converging.” 📰 Full Story →
 Novel embolization-on-a-chip model allows testing various embolic agent classes to treat liver cancer
Novel embolization-on-a-chip model allows testing various embolic agent classes to treat liver cancer
Terasaki Institute for Biomedical Innovation, Medical Xpress, 9/4/2025
“Dr. Vadim Jucaud’s lab at the Terasaki Institute has developed a human vascularized liver cancer-on-a-chip model to evaluate vessel remodeling and cell death in response to embolic agents. This novel platform reflects the microenvironment of liver tumors, particularly a functional and perfusable microvasculature that can be embolized.”
“’A vascularized cancer-on-a-chip offers a human-relevant, ethically sound alternative to animal testing, aligning with the growing push for non-animal technologies in preclinical drug development,’ said Dr. Vadim Jucaud, Principal Investigator.” 📰 Full Story →
 Animal-free drug discovery is closer with QSP
Animal-free drug discovery is closer with QSP
Drug Target Review, 9/5/2025
“Quantitative Systems Pharmacology (QSP) is rapidly moving from niche innovation to a standard tool in drug development. In the hands of researchers, QSP can simulate complex drug–disease interactions, predict human responses before clinical trials and guide more ethical, efficient research strategies.”
“ . . . these models are routinely used to assess target feasibility, identify optimal dosing strategies and predict clinical outcomes based on preclinical data. ‘These insights can shape the design of future experiments, reduce the need for animal testing and better position candidates for success later in development,’….”
“ . . . in certain contexts, QSP can ‘reduce or even replace the need for animal studies’, advancing both ethical imperatives and scientific precision . . . The aim is to build models that mirror human disease and therapeutic response closely enough to guide confident, evidence-based decisions – even in the absence of animal testing.” 📰 Full Story →
 From Policy to Practice: How Human-Based In Vitro Models Are Reshaping Discovery
From Policy to Practice: How Human-Based In Vitro Models Are Reshaping Discovery
Susan Harrison Uy, Technology Network, 9/9/2025
“For decades, the use of animals in drug discovery was viewed as a scientific necessity – sometimes even a gold standard. Mouse models, in particular, became synonymous with progress. But today, that standard is shifting quickly. In just a matter of months, we’ve seen a wave of landmark announcements that collectively mark a new chapter in preclinical science . . . They reflect a global structural shift, one that signals the end of animal-first thinking in biomedical research. ‘These policy changes aren’t aspirational, they’re a recognition that the old models aren’t working,’ said Cameron Ferris, PhD, COO and cofounder at Inventia Life Science. The industry knows it needs more predictive systems.’”
“ . . . the scientific community is increasingly embracing human-based in vitro models, specifically 3D phenotypic cell models of disease. These approaches aim to recapitulate key aspects of human biology, including tissue-specific architecture, cell–cell interactions, extracellular matrix dynamics and immune components, all within a controllable and screenable format.”
“Importantly, human-based in vitro models don’t just offer an alternative, they unlock new kinds of experimentation. When you’re no longer constrained by species differences or the ethical and logistical limitations of animal research, you can begin to ask more meaningful biological questions….” 📰 Full Story →
 Lilly launches AI-powered platform to accelerate drug discovery
Lilly launches AI-powered platform to accelerate drug discovery
Reuters, 9/9/2025
“Drugmaker Eli Lilly said on Tuesday it is launching an artificial intelligence and machine learning platform that provides biotech companies access to drug discovery models trained on years of its research data. Drug developers are increasing adoption of AI technologies for discovery and safety testing to get faster and cheaper results, in line with an FDA push to reduce animal testing in the near future.”
“Lilly’s platform, TuneLab, consists of AI models which include proprietary data obtained at a cost of over $1 billion . . . Privately held companies Circle Pharma and insitro said they are partnering with Lilly for TuneLab. Circle will be using Lilly’s platform to develop cancer therapies while insitro will build new AI models that will be used by TuneLab for the discovery of small molecule therapies.” 📰 Full Story →
 Genoskin raises $8.7M to advance human skin models as animal testing alternative
Genoskin raises $8.7M to advance human skin models as animal testing alternative
Cate Lawrence, TECH.EU, 9/16/2025
“Genoskin, a Contract Research Organisation (CRO) developing ex vivo human skin platforms capable of testing injected drugs and implanted medical devices, today announces it has raised $8.7 million in its first funding round. Genoskin provides a scalable and sustainable alternative to animal testing.”
“By leveraging donated human skin and its proprietary preservation technology, the company provides live immunocompetent *ex vivo *platforms that remain viable for up to seven days post-surgery, enabling more predictive, human-relevant testing than traditional animal or engineered models. The models generate large-scale datasets through multiomics approaches, which are further analysed using AI and advanced bioinformatics to deliver meaningful insights into the toxicity and efficacy of tested products in humans.” 📰 Full Story →
 Combining Organs-on-Chips with CRISPR
Combining Organs-on-Chips with CRISPR
Caitlin Ryan, The Medicine Maker, 9/18/2025
“Once seen as a thing of the future, organ-on-a-chip (OoC) systems and CRISPR gene editing have now matured into tools that are being widely used and developed across the world . . . When used together, these systems make it possible to recapitulate patient-specific physiology and model multi-organ interactions in real time.”
“The combination of CRISPR and OoC also gives us the tools to engineer disease at a patient level, and functionally test how to reverse it. In other words, personalized medicine.” 📰 Full Story →
 NIH earmarks $87M for new organoid center as pivot away from animal testing continues
NIH earmarks $87M for new organoid center as pivot away from animal testing continues
Darren Incorvaia, Fierce Biotech, 9/25/2025
“The National Institutes of Health is continuing its mission to reduce the use of animals in drug testing by awarding $87 million in contracts to launch the Standardized Organoid Modeling (SOM) Center, the agency announced in a Sept. 25 release. The funding, spread out over three years, will support the SOM Center as it takes root at the Frederick National Laboratory for Cancer Research, a Maryland facility of NIH’s National Cancer Institute (NCI).”
“‘By creating standardized, reproducible and accessible organoid models, we will accelerate drug discovery and translational science, offering more precise tools for disease modeling, public health protection and reducing reliance on animal models,’ NIH Director Jay Bhattacharya, Ph.D., said in the release.” 📰 Full Story →
 Breathtaking breakthrough: Lung-on-a-chip defends itself
Breathtaking breakthrough: Lung-on-a-chip defends itself
Georgia Institute of Technology, AAAS, 9/24/2025
“Lung-on-a-chip platforms provide researchers a window into organ behavior. They are about the size of a postage stamp, etched with tiny channels and lined with living human cells. Roy and Singh’s innovation was adding a working immune system — the missing piece that turns a chip into a true model of how the lung fights disease. Now, researchers can watch how lungs respond to threats, how inflammation spreads, and how healing begins.”
“For decades, lung research has relied on animal models. But mice don’t get asthma like children. Their bodies don’t mount the same defenses. ‘Five mice in a cage may respond the same way, but five humans won’t,’ Singh explained. ‘Our chip can reflect that difference. That’s what makes it more accurate, and why it could dramatically reduce the need for animal models.’” 📰 Full Story →
 Skin in the game: CTIBiotech pioneers “monumental leap” for cosmetics testing
Skin in the game: CTIBiotech pioneers “monumental leap” for cosmetics testing
Beatrice Wihlander, personal care insights, 9/25/2025
“CTIBiotech (cell, tissue, and innovation) has been showcasing its computer-connected, lab-grown, human bioprinted skin technology” that “is the ‘first of its kind’ and features a functional sensory nervous system resembling human skin. The primary purpose of lab-grown skin is to reduce reliance on animal testing while enhancing the accuracy and speed of cosmetics and fragrance testing processes.”
“‘Our computer-connected bioprinted skin represents a monumental leap forward, offering a more ethical, efficient, and predictive platform for the cosmetics industry. We believe this technology will accelerate the development of safer and more effective products and inspire a new generation of scientific inquiry into human biology,’….”
“The technology is a part of new approach methodologies (NAM), which aims to end animal testing. Cruelty-free beauty has recently seen a surge, especially in Europe, as consumers demand ethical personal care.” 📰 Full Story →
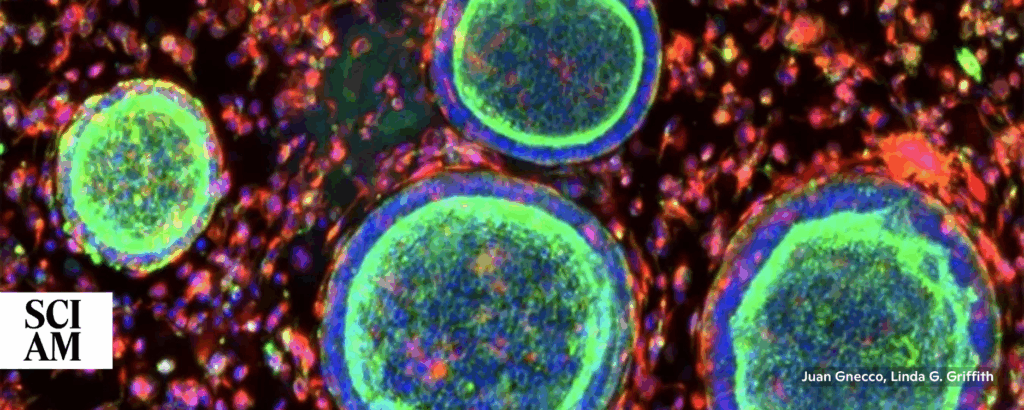 Lab-Made Mini Organs Could Transform Female Reproductive Medicine
Lab-Made Mini Organs Could Transform Female Reproductive Medicine
Cassandra Willyard & Nature Magazine, Scientific American, 9/27/2025
“ . . . researchers are using organoids to study female reproduction, an area in which animal models can be especially limited. Lab mice, for example, don’t menstruate. And their placentas don’t develop in the same way as human placentas do. That challenge, along with a historical lack of funding for women’s health research, has left basic questions unanswered.”
“Organoids of the placenta, endometrium, ovary and vagina could help to reveal how these organs function, and what happens when things go awry.” 📰 Full Story →
 Tiny models, powerful insights: how organoids are driving precision oncology
Tiny models, powerful insights: how organoids are driving precision oncology
Drug Target Review, 09/30/2025
“Organoid models are reshaping cancer research by replicating the intricate structures and diversity of human tumours. Unlike conventional flat cell cultures or animal models, organoids capture the true complexity of tumour growth, drug resistance and immune responses.”
“A team from Peking University People’s Hospital has published a new review in Cancer Biology & Medicine. Their work outlines how organoid models are revolutionising cancer research by providing realistic, patient-derived systems to test therapies and accelerate vaccine development . . . When combined with technologies such as microfluidics, single-cell sequencing and proteomics, these mini-tumours provide a living laboratory to test drugs, study tumour evolution and even design personalised cancer vaccines.”
“By merging patient-derived biology with high-throughput and high-resolution technologies, organoids offer researchers the means to move from general cancer models toward individualised treatment strategies . . . Clinicians can now use organoids to guide therapy choices and reduce exposure to ineffective drugs, while researchers gain a platform to explore drug resistance and identify biomarkers with precision. Pharmaceutical pipelines may also become faster and less costly as organoids reduce reliance on animal testing and streamline early-stage trials.” 📰 Full Story →
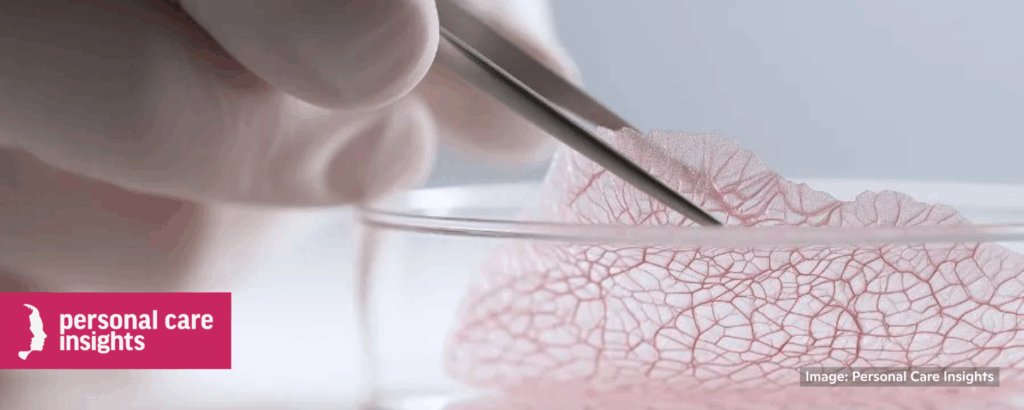 NIH organoid center to aid regulatory and industry acceptance of cruelty-free cosmetics testing
NIH organoid center to aid regulatory and industry acceptance of cruelty-free cosmetics testing
Mieke Meintjes, personal care insights, 9/30/2025
“The National Institutes of Health (NIH) is opening a ‘first of its kind’ Standardized Organoid Modeling (SOM) center that will influence how beauty companies test products by providing standardized, cruelty-free alternatives to animal testing. The center is designed for biomedical research but will directly benefit personal care companies seeking animal-free safety testing.”
“Organoids hold fantastic potential in helping the cosmetics industry transition away from animal testing, whether in the R&D phase or for regulatory-required testing for safety purposes,’ . . . organoids of skin, eye, liver, and other tissues are directly relevant to safety assessments needed in cosmetics.” 📰 Full Story →
 Take Action: Support The HEARTS Act
Take Action: Support The HEARTS Act
Ask your members of Congress to support The Humane and Existing Alternatives in Research and Testing Sciences Act of 2025 (“HEARTS Act of 2025”) (H.R. 1291). This bill aims to require that, in all NIH-funded research, nonanimal testing methods be prioritized when feasible. It also seeks to establish a National Center for Alternatives, and mandate improved reporting of animal use across federally-funded research. ⚠️ Take Action Now →
Share this news compilation on X or Bluesky.
Or share this link on Facebook or anywhere else:
riseforanimals.org/news/sci-news-sept-2025