While the animal research industry continues to breed, buy, cage, torture, and kill sentient beings, progressive scientists are busy proving that human-relevant science is not only possible but, in fact, better for us all. Highlights in science from the last month are below.
 Backed by Big Pharma, NIH foundation launches program to push animal testing alternatives across regulatory finish line
Backed by Big Pharma, NIH foundation launches program to push animal testing alternatives across regulatory finish line
Darren Incorvaia, Fierce Biotech, 8/1/2025
“The Validation and Qualification Network (VQN) is an initiative of the Foundation for the National Institutes of Health (FNIH), a congressionally mandated nonprofit that supports the mission of the NIH. The network, publicly announced July 24, brings together dozens of partners from regulators, like the FDA and European Commission, to Big Pharmas and CROs, such as Sanofi, Novo Nordisk, GSK and Charles River Laboratories.”
“ . . . the VQN sent out a call for new alternative method (NAM) developers to send in their ideas for network consideration. Submitted proposals will be reviewed on a rolling basis until Dec. 31, with the goal of choosing four projects in this first round of proposals, according to the notice. Submitted projects should ‘clearly define how their proposed concept may reduce, replace or refine the use of live animals,’ the network said….”
“ . . . the VQN will be ‘to take new approach methodologies that are well validated, or at least well on the pathway to validation, and push them towards acceptance for use in regulatory packages,’….” 📰 Full Story →
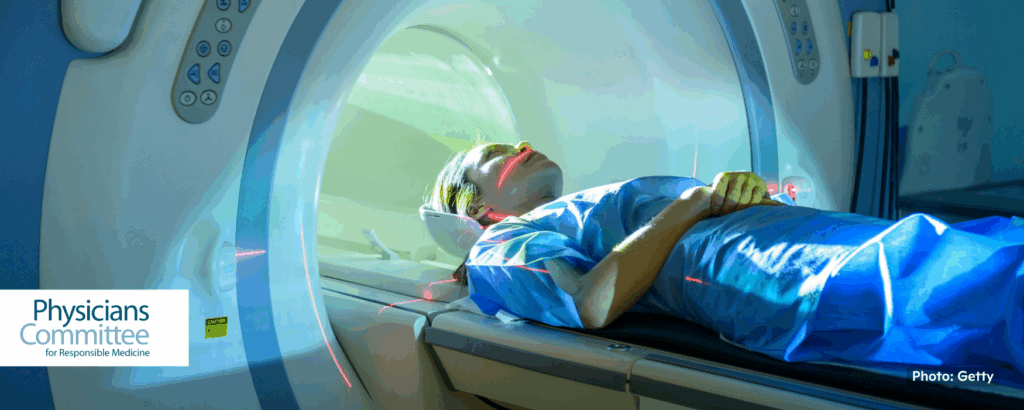 World’s Largest Human Imaging Study Opens the Door for a Better Understanding of Diseases
World’s Largest Human Imaging Study Opens the Door for a Better Understanding of Diseases
Physicians Committee for Responsible Medicine, 8/1/2025
“UK Biobank, one of the world’s most comprehensive datasets of human biological and health information, has scanned the bodies of 100,000 people . . . By combing these scans and the initial data collected, scientists can better understand how organs change and how diseases, including dementia and heart disease, develop.”
“The global availability of data at the UK Biobank has already resulted in more than 1,300 peer-reviewed papers with results showing improved patient care. While animal experimentation is fraught with scientific and ethical problems, human-based methodologies, including advanced imaging, allow researchers to see inside the human body and learn more about diseases at multiple stages without the use of animals.” 📰 Full Story →
 Lab-Grown Mini-Brains with Blood Vessels: A Breakthrough for Neurodrug Success
Lab-Grown Mini-Brains with Blood Vessels: A Breakthrough for Neurodrug Success
Nidhi Goyal, Industry Tap, 8/5/2025
“In a groundbreaking development, scientists have successfully grown a miniaturized brain-like structure, complete with functional blood vessels, right in the lab. This innovation could dramatically improve the success rate of neurological drug trials and finally tackle a major hurdle in neuroscience: why nearly 90% of neurodrugs fail in clinical trials.”
“Beyond its scientific precision, this lab-grown organoid also provides a humane and more accurate alternative to animal testing. It allows researchers to observe drug behavior in a setting much closer to the human brain, which significantly improves predictability and reduces costly failures in the later stages of drug development.” 📰 Full Story →
 Heart-In-A-Jar Organ Model Earns Regulatory Respect After 25 Years In The Making
Heart-In-A-Jar Organ Model Earns Regulatory Respect After 25 Years In The Making
Clinical Leader, 8/7/2025
“‘Animal hearts differ dramatically from human hearts. For example, rodents have heart rates of 600 beats per minute versus 70 beats per minute in humans. So, we set out to build a system to replicate human-specific cardiac function.’”
“‘It’s literally a beating heart chamber sitting in a jar. It takes about a week to convert human pluripotent stem cells into beating cardiomyocytes, which become fundamental building blocks for a working heart. And because they are coming from a donor, they are genetically and immunologically identical to that person. You’ve got only one heart sitting in your chest, and you don’t want to use it for testing unknown drugs. So, we can use these cardiac surrogates to represent your heart to test new drugs. We can optimize the dosages, all sort [sic] of things that you don’t want to do with your own body. So, it’s not just a heart-in-a-jar. It’s a smarter way to predict human outcomes. We’re not just replacing animals; we’re replacing guesswork.’” 📰 Full Story →
 IU receives $16 million national grant to study Alzheimer’s using human brain models
IU receives $16 million national grant to study Alzheimer’s using human brain models
Benjamin Thorp, WFYI Indianapolis, 8/8/2025
“Indianapolis will be home to one of two federally backed centers studying Alzheimer’s disease using stem-cell models of the human brain. Researchers at the IU School of Medicine have received a five-year, $16.5 million grant to better understand the underlying causes of Alzheimer’s.”
“What will set the research at IU apart is the use of so-called ‘brain organoids’ – human stem cells organized into three-dimensional models meant to mimic aspects of the human brain on a small scale. Basically, small human lab brains.” 📰 Full Story →
 TU Berlin adopts animal-free cell culture and 3D printing methods in training
TU Berlin adopts animal-free cell culture and 3D printing methods in training
Lucia Gartner, 3Printr.com, 8/12/2025
“The Technical University of Berlin is introducing a laboratory course in its Master’s program in Biotechnology that operates entirely without animal-derived products or methods . . . students will learn to apply molecular biology techniques without relying on traditional animal-based reagents. The aim is to establish methods that offer both ethical and scientific benefits. Animal products such as fetal bovine serum (FBS) are considered not only ethically problematic but also prone to quality fluctuations that can distort research results.”
“Another focus is the use of 3D printing to create human organ models from patient-derived cells. These models can replace conventional animal experiments in toxicological and pharmacological studies and allow for more accurate predictions of drug efficacy in humans.” 📰 Full Story →
 3D-Printed Kidney Tumors Open New Pathways for Targeted Cancer Therapies
3D-Printed Kidney Tumors Open New Pathways for Targeted Cancer Therapies
Scienmag, 8/12/2025
“In a groundbreaking advancement in cancer research, scientists at Tsinghua University have pioneered a novel technique to culture kidney tumors in laboratory settings directly derived from patient cells. This cutting-edge approach . . . leverages sophisticated 3D bioprinting technology to fabricate renal cell carcinoma (RCC) organoids that retain the distinct biological hallmarks of the original tumors. By integrating multiple cellular components, including tumor cells and vascular-like structures, the research team has generated a dynamic cellular microenvironment that closely mirrors in vivo conditions, offering unprecedented accuracy for therapeutic testing and cancer biology exploration.”
“Traditional models used to study RCC and evaluate treatment efficacy have long suffered from significant drawbacks. Conventional two-dimensional cell cultures and animal models often fail to replicate the intricate heterogeneity and microarchitecture of human tumors, which critically influences therapy responses and disease progression.” 📰 Full Story →
 Improving gene therapy safety with human kidney organoids
Improving gene therapy safety with human kidney organoids
News Medical Life Sciences, 8/19/2025
“Gene therapy holds great promise for treating serious genetic diseases such as Duchenne muscular dystrophy (DMD). However, unexpected toxic side effects, including patient deaths in some DMD trials, have raised major safety concerns. These risks are often missed in animal studies, revealing a critical flaw in current preclinical testing methods. In fact, fewer than 15% of drugs that enter clinical trials ever receive FDA approval, largely because traditional lab models fail to predict how the human body will respond to the treatment.”
“To address this significant gap, our study used human stem cell-derived kidney organoids-lab-grown mini kidneys-to test the safety of gene editing delivered by adeno-associated virus (AAV), a common tool in clinical trials. We found that AAV2 (one of several variants of the AAV virus currently used to deliver gene therapy treatment) caused significant harm to kidney cells by triggering inflammation, DNA damage, and fibrosis. This damage occurred through the NFκB pathway, even without any gene editing taking place . . . These results show that human-centric lab models such as organoids are vitally needed to detect hidden risks and improve the safety of gene therapies before they reach patients.” 📰 Full Story →
 ‘Minibrains’ reveal secrets of how key brain cells form in the womb
‘Minibrains’ reveal secrets of how key brain cells form in the womb
Nicoletta Lanese, LiveScience, 8/24/2025
“Immune cells in the human brain may be critical to orchestrating the organ’s development in the womb because they trigger a dramatic increase in an important type of nerve cell, new research suggests.”
“ . . . researchers have uncovered a force that drives [these key cells, known as inhibitory interneurons] to multiply in the developing human brain — and they say it may be unique to our species. ‘That’s why we cannot use traditional animal models,’ study co-author Diankun Yu, an assistant researcher in pediatrics at the University of California, San Francisco (UCSF), told Live Science. To uncover this mechanism that may unfold only in the human brain, the researchers developed an organoid — a miniature 3D structure, grown from stem cells, that mimics a full-size structure found in the human body.” 📰 Full Story →
 One Health world: Closing the nutrition science gap with organ-on-chip tech
One Health world: Closing the nutrition science gap with organ-on-chip tech
Venya Patel, Nutrition Insight, 8/25/2025
“The researchers state that [organ-on-chip (OoC)] models replicate human-specifics that only conventional models can approximate. ‘For example, advanced intestine-on-chip systems reproduce peristaltic motion, fluid flow, gut microbiota interactions, and oxygen gradients under controlled conditions, enabling the detailed study of nutrient absorption, barrier integrity, and host-microbiota crosstalk in a human-relevant setting,’ . . . ‘This is critical because rodent gut physiology and microbiota composition differ substantially from humans, making translation of findings uncertain.’”
“ . . . OoCs could help uncover the biggest blind spot in current nutrition research as animal studies or static cell cultures are insufficient. ‘They do not adequately reproduce key features . . . As a result, many mechanisms by which foods or contaminants influence human health remain poorly understood. OoC systems help to fill this gap by recreating human-relevant conditions and by linking multiple organs together. For example, gut-liver and gut-brain chips can reveal how dietary compounds or microbiota-derived metabolites are processed and how they impact systemic health — processes that traditional models routinely miss’ . . . ‘When combined with other NAMs, such as in silico modeling and omics sciences, OoC systems could provide a powerful toolkit to reconstruct and interrogate these dynamic networks with a precision not possible in animals. In this way, they have the potential to uncover mechanisms that remain hidden with traditional methods, offering a more predictive understanding of how nutrition impacts human health.’” 📰 Full Story →
 Israeli hospital developed lab-grown kidneys that survived for 34 weeks
Israeli hospital developed lab-grown kidneys that survived for 34 weeks
TOI Lifestyle Desk, Times of India, 8/28/2025
“Sheba Medical Centre along with the help of Tel Aviv University has successfully created a synthetic 3D organ culture or organoid that was the first to survive beyond 34 weeks, reported the South China Morning Post.”
“ . . . kidney organoids help researchers model kidney disease, making it easy for them to understand the underlying mechanisms and allowing timely interventions. This also aids in reducing the dependency of drug tests being conducted on mice.” 📰 Full Story →
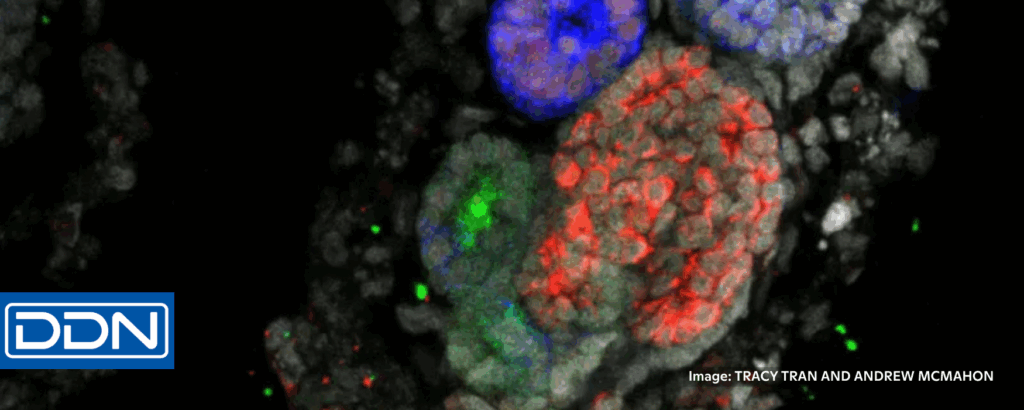 Kidney disease research grows in a dish
Kidney disease research grows in a dish
Marilyn Perkins, DDN, 8/26/2025
“More than one in seven adults in the US suffer from chronic kidney disease, which ranks among the leading causes of death in the country. But until now, research on kidney disease has faced major hurdles. The kidney is a highly complex organ composed of numerous specialized cell types, making it difficult to replicate using traditional cell culture. And . . . [animal] models fall short for several reasons.”
“‘Animal models are mechanistically complex, so they’re hard to really get into and perturb and figure out exactly how a pathway is working,’ Freedman said. ‘Organoids are a little better in that regard. You can watch them. You can take things out of the system and see if they’re necessary or change components of the system in a way that’s more difficult to do in a living organism.’ Using this method, Freedman uncovered potential new ways to treat [polycystic kidney disease] . . . ‘I think we’ll see, increasingly, a choice presented between using animals and using organoids.’” 📰 Full Story →
 ‘Eye-on-a-chip’ reveals trigger for steroid-induced glaucoma
‘Eye-on-a-chip’ reveals trigger for steroid-induced glaucoma
David Nutt, Cornell Chronicle, 8/27/2025
“ . . . Cornell researchers have identified the signaling mechanism that triggers steroid-induced glaucoma by creating a 3D ‘eye-on-a-chip’ platform that mimics the flow of ocular fluids. ‘Steroid-induced glaucoma is a major clinical challenge. There’s no targeted therapy. We just say you are unlucky,” said senior author Esak (Isaac) Lee, assistant professor of biomedical engineering at Cornell Engineering. ‘There is a clear, unmet need to better understand, and prevent, this major side effect of the steroid in the clinics.’”
“Glaucoma is typically studied in animal models and simple 2D cell cultures, but those approaches often fail to capture the anatomical complexity and responsiveness of the human eye. The solution from Lee’s lab, which studies lymphatic systems in different types of organs, has been to create 3D in-vitro models that can reproduce the systems’ layered structures while isolating biological and biophysical factors, all in a highly reproducible and controlled manner.” 📰 Full Story →
 Take Action: Support The CARE Act
Take Action: Support The CARE Act
For far too long, the Animal Welfare Act (AWA) — the only federal law to establish even basic care standards for animals in labs and exhibitions — has excluded nearly all of the beings its name purposes to include. Cold-blooded animals, including fish, cephalopods, reptiles, and amphibians, have been deliberately left out of the law’s definition of “animal.”
The CARE Act would correct this failure, finally extending AWA recognition to tens of millions of sentient animals. Urge your members of Congress to support The CARE Act: ⚠️ Take Action Now →
Share this news compilation on X or Bluesky.
Or share this link on Facebook or anywhere else:
riseforanimals.org/news/sci-news-aug-2025